Cochlear announces launch of new cochlear implant
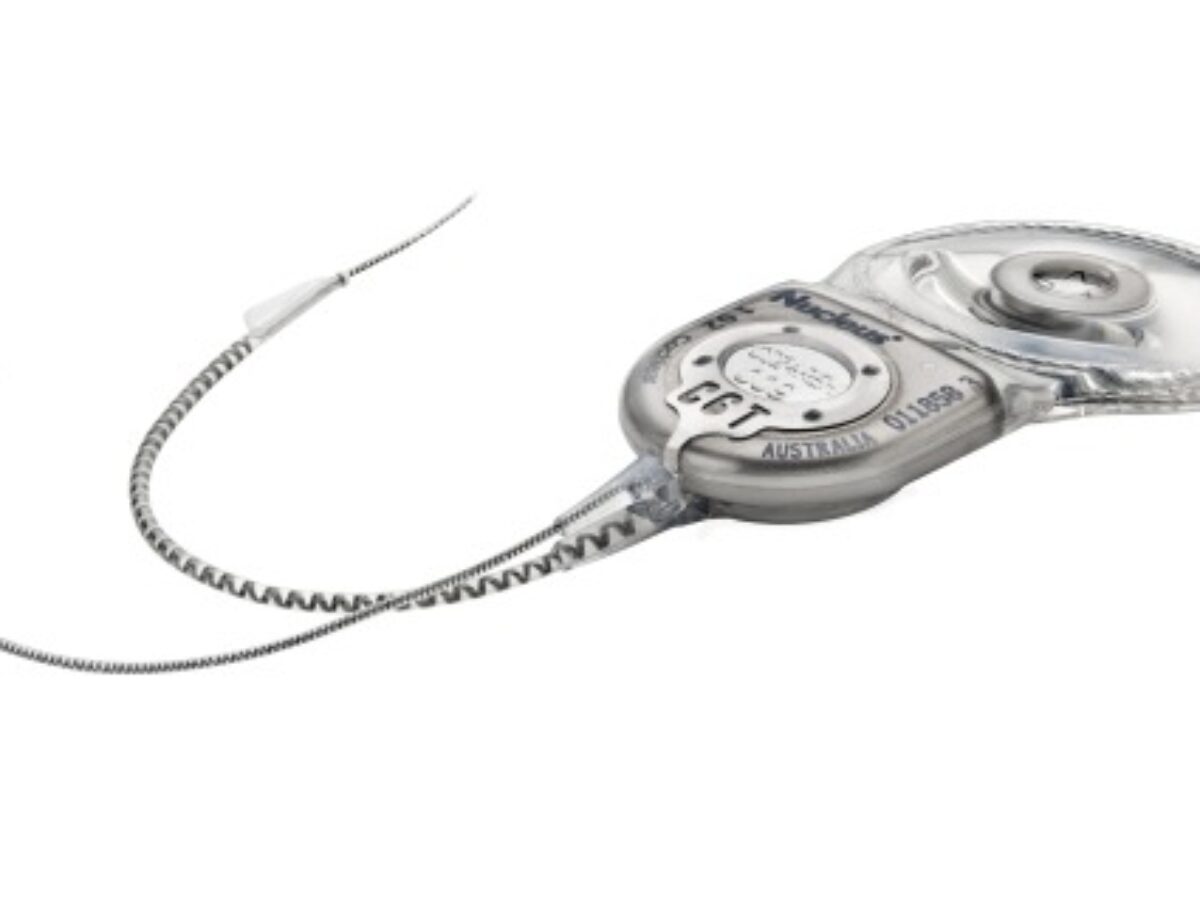
Medical device manufacturer Cochlear (ASX: COH) has announced the launch of its latest Nucleus Profile Plus Series cochlear implant, and its approval for sale in the United States by the FDA.
In a series of ASX announcements today the Sydney company announced commercial availability for the implant in Germany with other European countries to follow.
It also announced all important approval in the worlds’s biggest market for medical devices and said it “will commence an immediate US launch”.
The Profile Plus is a refinement of the company’s latest implant, which is the thinnest available on the market.
The latest development allows routine 1.5 and 3 Tesla magnetic resonance imaging (MRI) scans without the need to remove the device’s internal market.
Picture: Cochlear Profile Series
Subscribe to our free @AuManufacturing newsletter here.
Topics Manufacturing News
@aumanufacturing Sections
Analysis and Commentary Awards Defence Manufacturing News Podcast Technology Videos