Cortical Dynamics scores brain monitor approvals
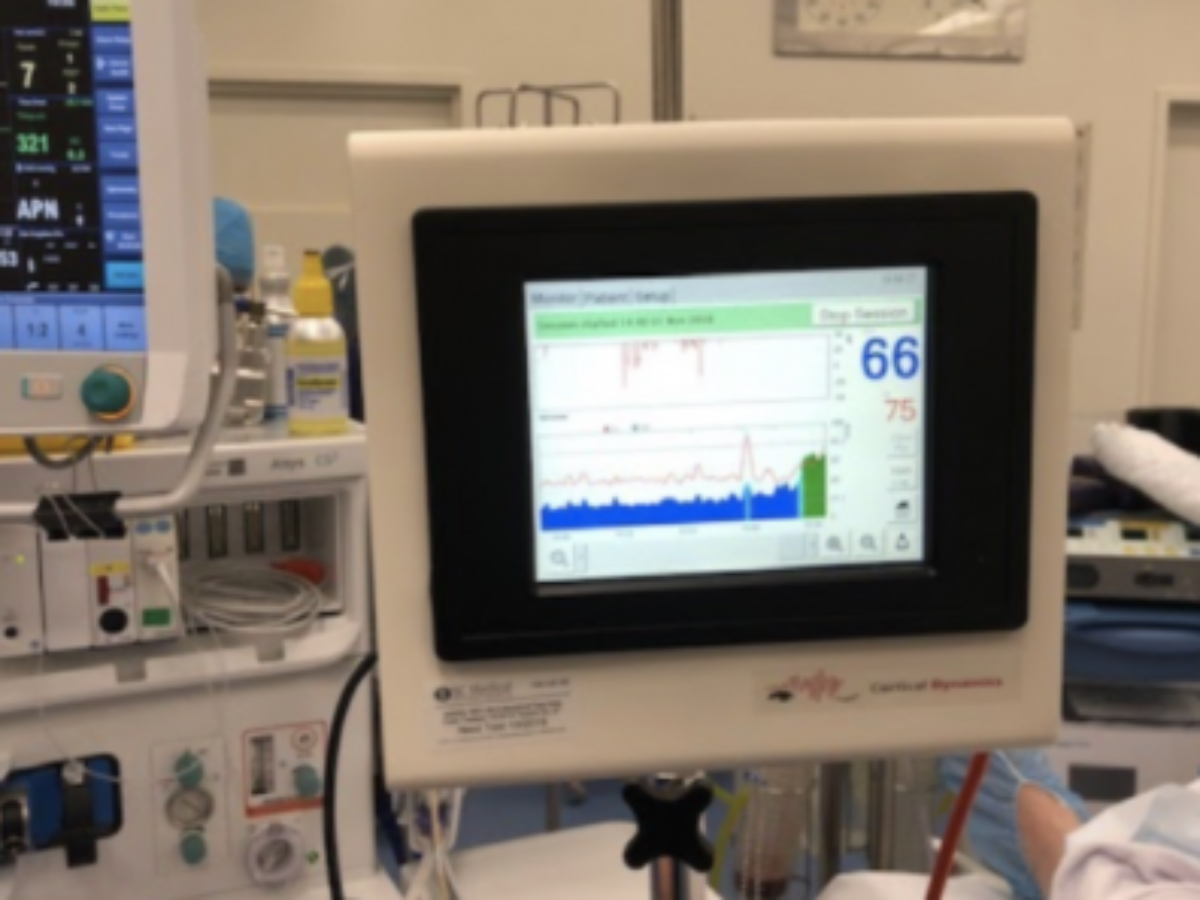
Medical device manufacturer has won regulatory approval to market its Brain Anaesthesia Response Monitor (BARM, pictured) in South Korea, building on its existing approvals in Europe and through Australia’s Therapeutic Goods Administration (TGA).
The Perth company won approval from Korea’s Ministry for Food and Drug Safety (KMFDS).
The company’s overseas distributors are Globaluck in South Korea and Innomed Benelux B.V in Europe.
Based on intellectual property originally developed at Swinburne University in Melbourne, the BARM system detects the effect of anaesthetic agents on brain activity, aiding anaesthetists in keeping patients optimally anaesthetised.
Claimed to be ‘fundamentally different to all other currently available devices’, BARM monitors the brain’s rhythmic electrical activity or EEG, relating that to the brain’s physiological state.
Cortical Dynamics was the winner of the Advanced Manufacturing category at the Australian Technologies Competition (ATC) awards.
Picture: Cortical Dynamics
Subscribe to our free @AuManufacturing newsletter here.
Topics Manufacturing News
@aumanufacturing Sections
Analysis and Commentary Awards Defence Manufacturing News Podcast Technology Videos










