Recce Pharmaceuticals to test for burn healing
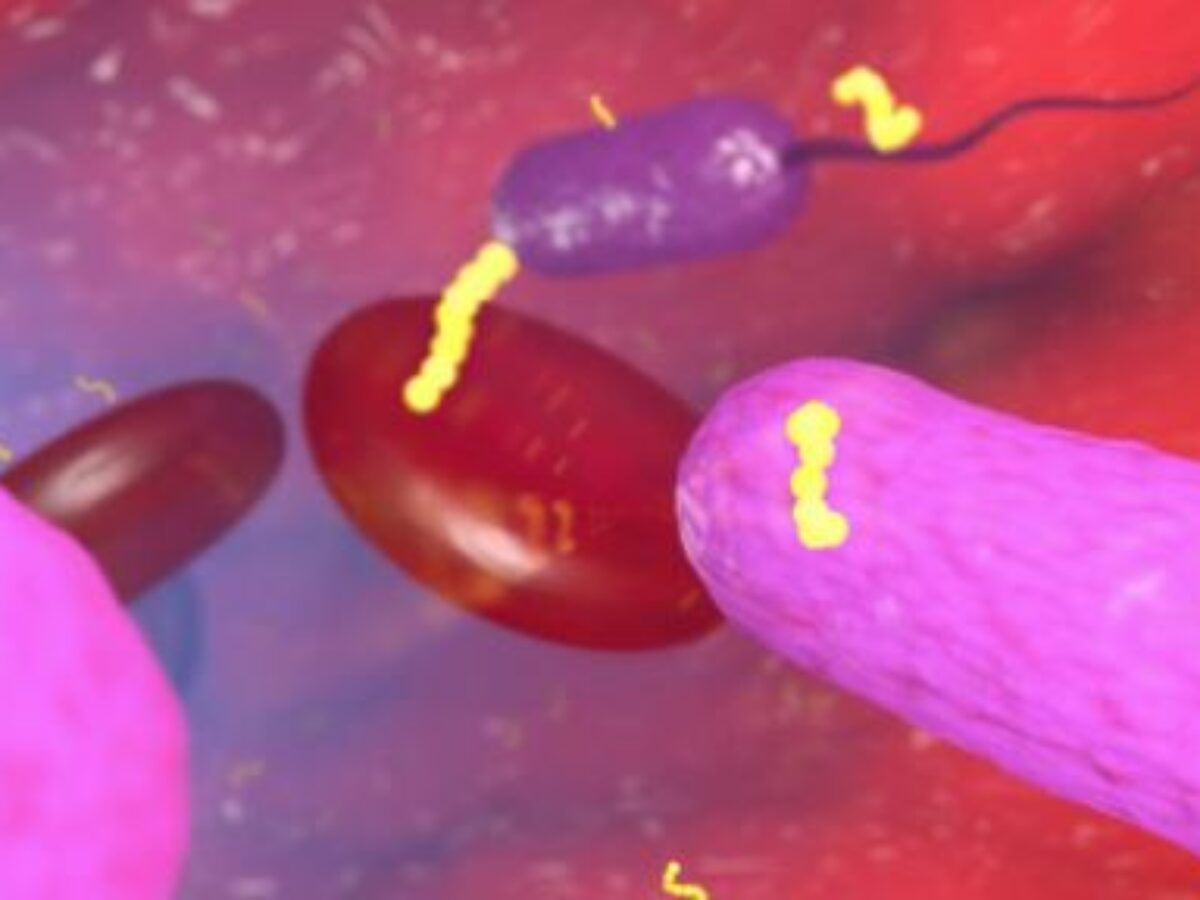
Anti-infective drug development company Recce Pharmaceuticals is to test its lead compound RECCE 327 (R327) in a study of topical burn healing in humans.
The company has been registered in the Australian New Zealand Clinical Trial Registry (ANZCTR), one of the final stages before Phase I/II clinical trials may begin.
The Company’s clinical trial is registered under ‘Proof of Concept Study of RECCE 327 Topical Antibiotic Therapy for Infected Burn Wounds in Adults’ and involves 30 patients to assess safety and efficacy of R327.
Over 14 days, 10 patients will receive R327 daily while a further 20 receive treatment three times per week.
RECCE 327 was originally developed for the treatment of blood infections and sepsis derived from E. coli and S. aureus bacteria – including their superbug forms.
The US Food and Drug Administration has given RECCE 327 Qualified Infectious Disease Product designation under the Generating Antibiotic Initiatives Now (GAIN) Act.
This paves the way for fats track registration of the drug.
Picture: Recce Pharmaceuticals
Subscribe to our free @AuManufacturing newsletter here.
Topics Technology
@aumanufacturing Sections
Analysis and Commentary Awards casino reviews Defence Gambling Manufacturing News Online Casino Podcast Technology Videos