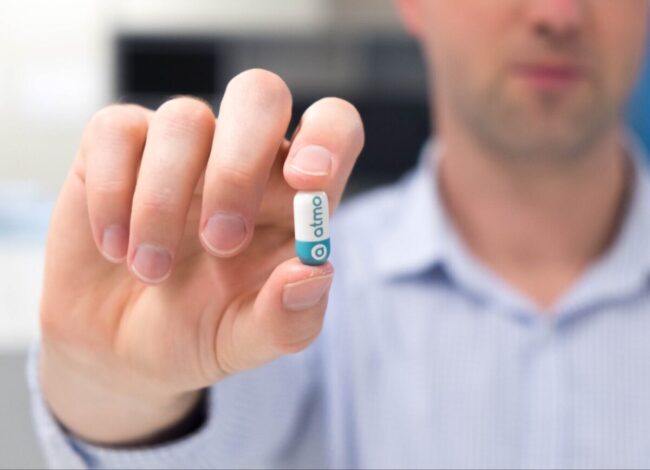
RMIT transfers all IP for gas capsule to Atmo Biosciences in exchange for equity
An ingestible capsule used to monitor gut health markers has taken another small step towards commercialisation, with RMIT University transferring IP ownership to Atmo Biosciences. Digital health business Atmo Biosciences is commercialising a capsule that it says “measures gases as it travels through the gastrointestinal tract and transmits the data wirelessly” and which was first…