Tiny solution to dissolving microplastic pollution
By Andrew Spence
The lead researchers from the University of Adelaide in South Australia say by-products created in the breakdown process could also be harnessed as an energy source for microorganisms that the polluting plastics currently plague.
Although microplastics pollution in household wastewater and global aquatic systems is a known threat to marine life and human health, advanced technologies for efficient microplastics control and purification remain largely underdeveloped.
The Australian researchers are tackling this by using tiny coil-shaped carbon-based magnets laced with nitrogen to help boost generation of the reactive oxygen species to break the microplastics down.
“Microplastics absorb organic and metal contaminants as they travel through water and release these hazardous substances into aquatic organisms when eaten, causing them to accumulate all the way up the food chain” senior author Shaobin Wang, a professor of chemical engineering at the University of Adelaide said.
“Carbon nanosprings are strong and stable enough to break these microplastics down into compounds that do not pose such a threat to the marine ecosystem.”
Although often invisible to the naked eye, microplastics are omnipresent pollutants. Some, such as the exfoliating beads found in popular cosmetics, are simply too small to be filtered out during industrial water treatment. Others are produced indirectly, when larger debris like plastic bottles weather amid sun and sand.
To decompose the microplastics, the researchers had to generate short-lived chemicals called reactive oxygen species, which trigger chain reactions that chop the various long molecules that make up microplastics into tiny and harmless segments that dissolve in water.
Professor Wang said those intermediates and by-products could be harnessed as an energy source for microorganisms such as algae.
“If plastic contaminants can be repurposed as food for algae growth, it will be a triumph for using biotechnology to solve environmental problems in ways that are both green and cost efficient,” he said.
Reactive oxygen species are often produced using heavy metals such as iron or cobalt, which are dangerous pollutants in their own right and thus unsuitable in an environmental context.
The researchers found the carbon nanotubes to be a much greener solution.
Shaped like springs, the carbon nanotube catalysts removed a significant fraction of microplastics in just eight hours while remaining stable themselves in the harsh oxidative conditions needed for microplastics breakdown.
The coiled shape of the carbon nanotubes increases stability and maximises reactive surface area. As a bonus, by including a small amount of manganese, buried far from the surface of the nanotubes to prevent it from leaching into water, the minute springs became magnetic.
“Having magnetic nanotubes is particularly exciting because this makes it easy to collect them from real wastewater streams for repeated use in environmental remediation,” said Dr Xiaoguang Duan, a chemical engineering research fellow at Adelaide who also co-led the project.
As no two microplastics are chemically identical, the next steps for the researchers will be to ensure the nanosprings work on microplastics of different compositions, shapes and origins.
They also intend to continue to rigorously confirm the non-toxicity of any chemical compounds occurring as intermediates or by-products during microplastics decomposition.
The study has been published in the journal Matter and was supported by the Australian Research Council, the National Natural Science Foundation of China and the Science and Technology Program of Guangdong Province.
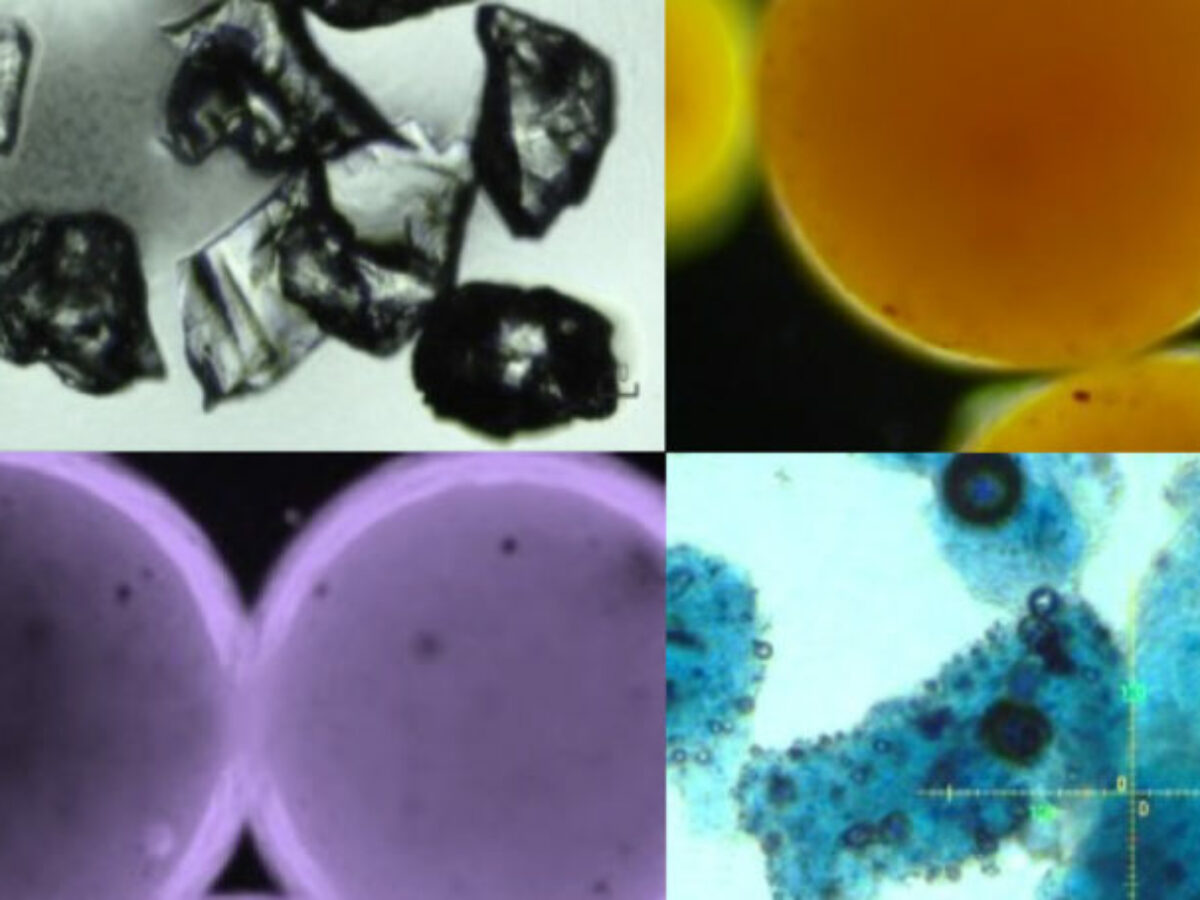
Picture: microplastic debris in cosmetics under optical microscopy (Credit: Dr.-Xiaoguang Duan)
This article originally appeared at The Lead SA.
Subscribe to our free @AuManufacturing newsletter here.
Topics Manufacturing News Technology
@aumanufacturing Sections
Analysis and Commentary Awards casino reviews Defence Gambling Manufacturing News Online Casino Podcast Technology Videos





