Vaxxas completes enrolment for needle-free vaccine patch trial
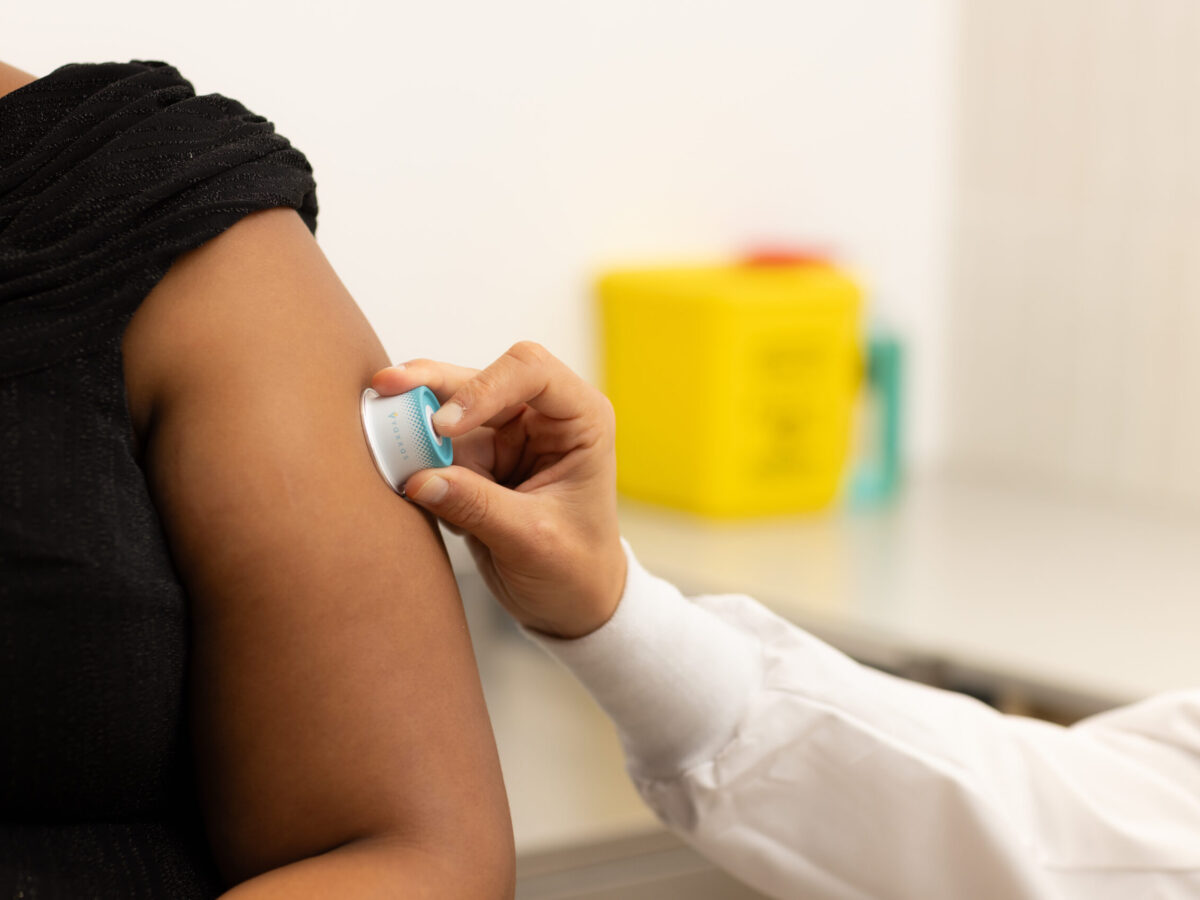
Brisbane biotechnology company Vaxxas has completed enrolment for testing a needle-free vaccine patch against avian influenza.
The Phase I trial, its largest clinical trial to date, enrolled 258 healthy participants aged 18-50 years across sites in Melbourne and Queensland to evaluate the company's high-density microarray patch (HD-MAP) technology.
The trial will assess both safety and immune response to the H7N9 influenza vaccine when delivered via the HD-MAP system compared to traditional needle and syringe methods. For the first time, Vaxxas will also test an adjuvanted vaccine formulation using its patch technology.
“In our prior clinical studies for seasonal influenza, we've demonstrated comparable immune responses to traditional vaccination with as little as one-sixth of the vaccine with no adjuvant by delivering the vaccine directly to the immune cells just below the skin surface,” said David Hoey, Vaxxas CEO.
The project received US$28.5 million in funding from the U.S. Biomedical Advanced Research and Development Authority (BARDA), with approximately 85 per cent used for the trial's enrolment phase.
Early studies indicated the HD-MAP system could offer advantages over traditional vaccination methods, including reduced cold-chain storage requirements and potential self-administration capabilities.
Research by the Avalere Group suggested that if 10 per cent of COVID-19 vaccinations had used HD-MAP technology, the pandemic in the United States could have been shortened by up to 150 days.
Initial results from the trial are expected in the first half of 2025.
Pictures: supplied

@aumanufacturing Sections
Analysis and Commentary Awards casino reviews Defence Gambling Manufacturing News Online Casino Podcast Technology Videos





