Next Science set to launch XPerience antimicrobial rinse
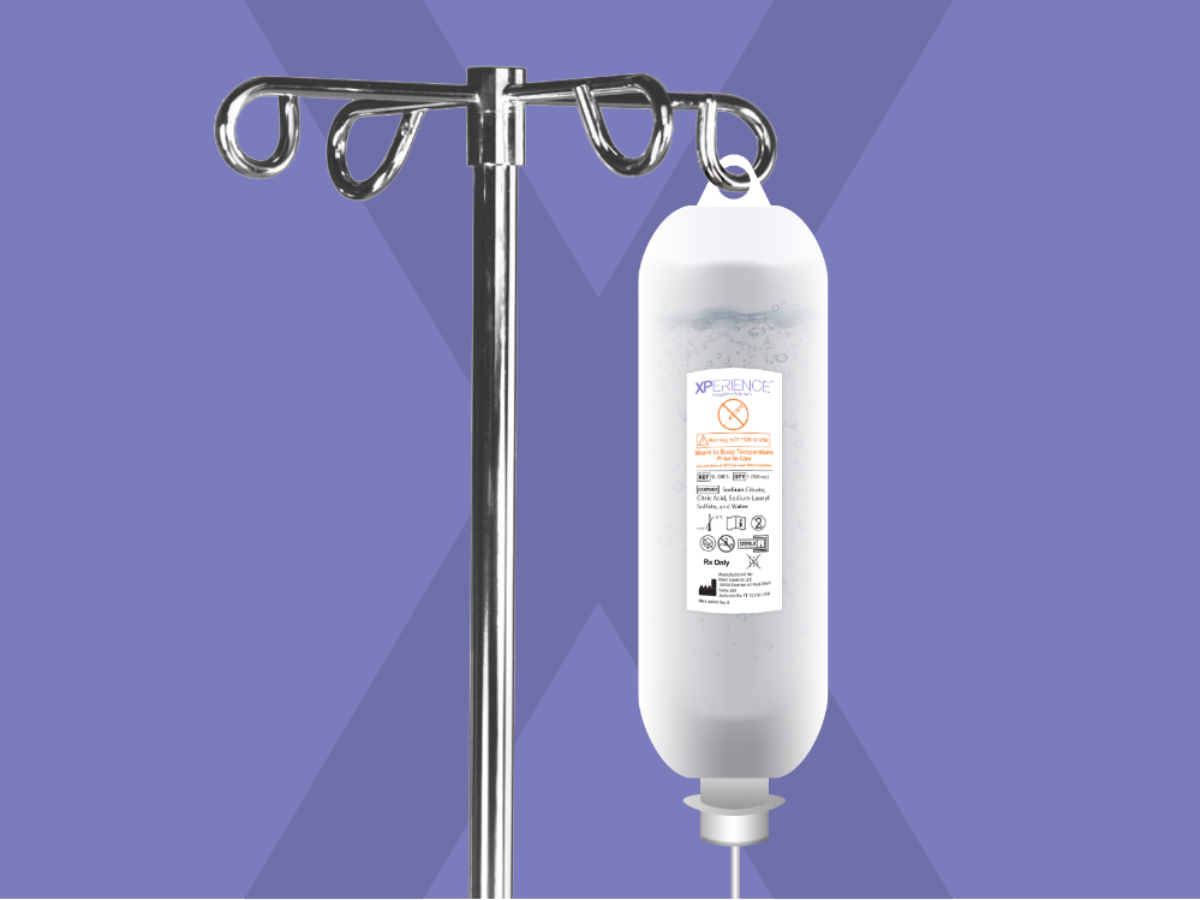
Wound care technology developer Next Science will begin commercial sales immediately of its XPerience No Rinse Antimicrobial Solution after it received US Food and Drug Administration approval to market the product.
The company’s shares soared 14.5 per cent yesterday on the news to $1.62, with the Sydney company claiming a US$15 billion market potential.
Next Science told investors: “XPerience enters the market as a single step application where the residual solution remains in the surgical site after closure and continues to help defend against pathogens for several hours, giving surgeons a simple and effective adjunct to help prevent surgical site infections and post-operative infections.”
XPerience is based on the company’s patented Xbio technology which attacks the biofilm that surrounds and protects bacteria.
The company will initially focus the product on surgeries of the shoulder, hip, knee and in trauma and podiatry.
Next Science managing director Judith Mitchell said XPerience reduced pathogens such as golden staph (MRSA) to fewer than one bacterium in a hundred million.
She said: “We expect the product will become a first choice in the battle to reduce surgical site infection.
“With an estimated 234 million surgical procedures undertaken globally per annum, XPerience provides an enormous opportunity to help reduce infection, antimicrobial resistance and save lives.”
The product also aims to reduce healthcare costs from postsurgical infections.
Surgery is the second largest cause of hospital acquired infection in the US and a major cause of complications and death.
Picture: Next Science
Subscribe to our free @AuManufacturing newsletter here.
Topics Technology
@aumanufacturing Sections
Analysis and Commentary Awards Defence Manufacturing News Podcast Technology Videos










