Vaxxas licenses RSV vaccine candidate
Needle free biotechnology company Vaxxas has announced that the United States National Institutes of Health (NIH) has granted the company a licence to a vaccine antigen candidate (DS2), designed for use in prophylactic vaccines against Respiratory Syncytial Virus (RSV).
RSV is a common contagious virus affecting the lungs and breathing passages which causes an estimated 33 million acute lower respiratory infection episodes per year.
Vaxxas’ worldwide licence from the NIH enables the company to develop the first needle-free, room-temperature stable RSV vaccine to enter clinical studies.
The DS2 RSV vaccine antigen candidate licensed by Vaxxas was developed by scientists at the NIH’s Vaccine Research Centre and the National Institute of Allergy and Infectious Diseases to prompt a more robust and durable immune response against RSV.
Vaxxas CEO David Hoey said: “Published preclinical results show the potential immunogenic advantages of this antigen candidate as the basis for an RSV vaccine to provide robust and durable protection.
“These potential advantages, coupled with the potential of the Vaxxas needle-free technology to eliminate the need for refrigerated distribution and enable self-administration, could offer a vaccine that makes a significant impact on the way we protect populations against this serious respiratory infection in the future.”
The Brisbane company intends to progress the needle-free RSV vaccine antigen candidate to a Phase I clinical study after completing preclinical development of the product.
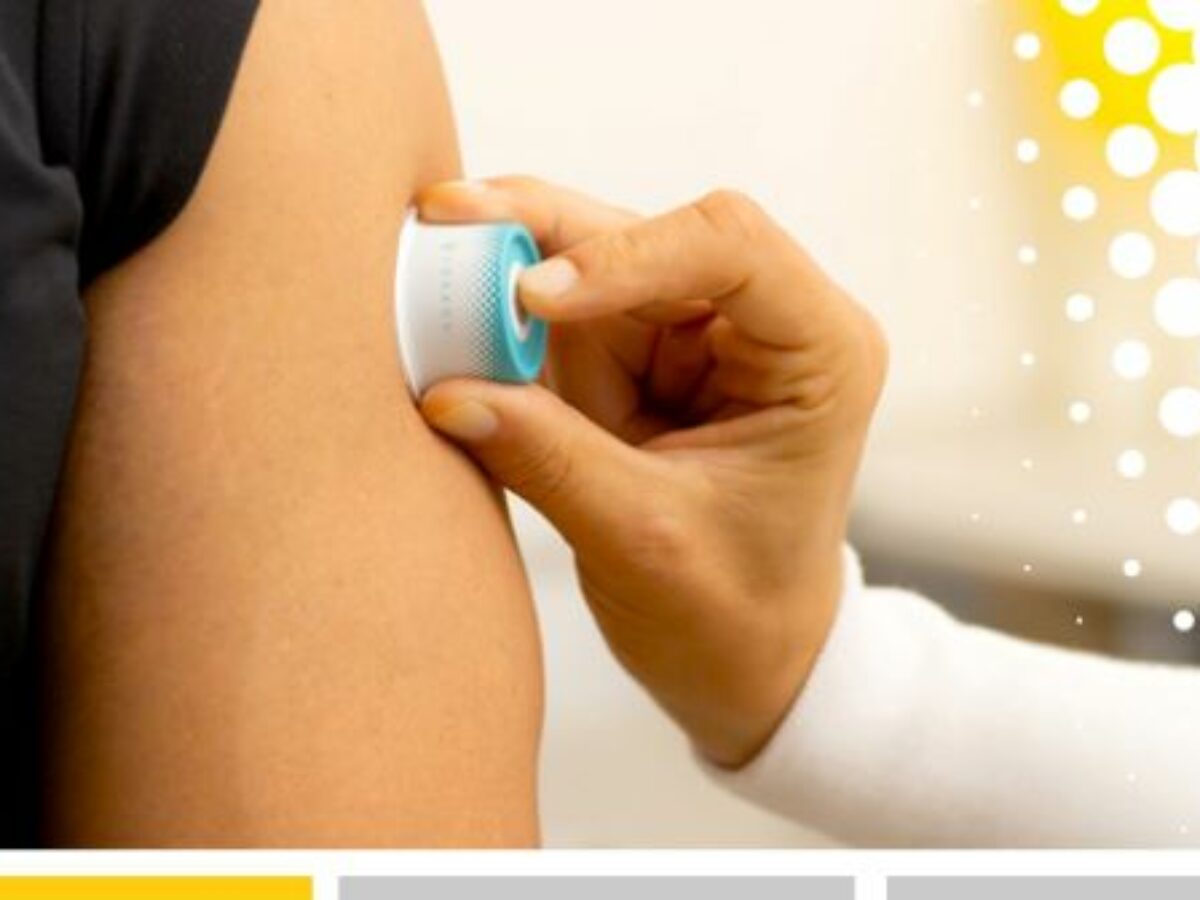
Vaxxas' needle-free technology uses a patch with thousands of vaccine-coated microprojections that is applied to the skin for a few seconds to deliver vaccine to the immune cells immediately below the skin surface.
The company's HD-MAP vaccine delivery platform has been used in five successful Phase I clinical trials, including a COVID-19 vaccine candidate, a HD-MAP delivered flu vaccine, and a measles and rubella vaccine.
With funding from the United States Biomedical Advanced Research and Development Authority (BARDA), the company is conducting its first US IND-enabled Phase I clinical study for a pre-pandemic influenza vaccine involving 258 participants in Australia.
Picture: Vaxxas needle free delivery technology
@aumanufacturing Sections
Analysis and Commentary Awards casino reviews Defence Gambling Manufacturing News Online Casino Podcast Technology Videos