Immuron booms on Travelan’s positive research result
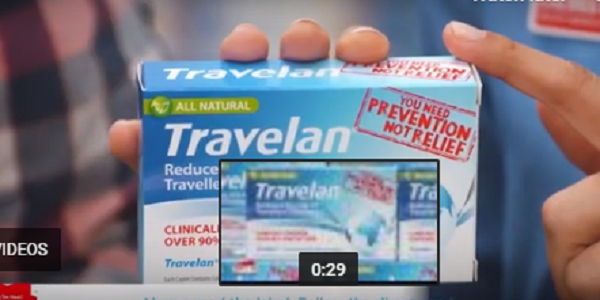
Drug developer Immuron’s Travelan anti-diarrhoea oral antibody product set the company share price alight this morning following positive news from clinical trials in the United States. Travelan, which is made in Melbourne and on sale through Chemist Warehouse, is a non-absorbable, safe product which is composed of anti-bacterial antibodies that boost the gut immune system.…